
Article | Ming Xinhui
1. Molecular Biology of Janus Kinase (JAK)
Immune and inflammatory responses are central biological processes in many diseases and are relevant to a wide range of diseases. Among the wide range of drug targets in the field of immunity, Janus Kinase (JAK) is an extremely important class of targets that plays a key role in the signaling and biological functions of non-receptor-type loribody kinases.The JAK family consists of four isoforms, namely, JAK1, JAK2, JAK3, and Tyk2. These proteins share many similarities in secondary structure and all contain four identical structural domains PERM-SH2-Pseudokinas-Kinase, allowing JAK proteins to play important roles in cell signaling.
Janus kinase is named after Janus, the two-faced god of ancient Roman mythology, because the kinase has two nearly identical phosphorylated structural domains: a functional Kinase domain and a Pseudokinase domain that is largely devoid of kinase activity. These two structural domains can modulate Janus kinase activity to some extent, making these proteins like the two-faced god Janus with two different properties (Figure 1) [1].
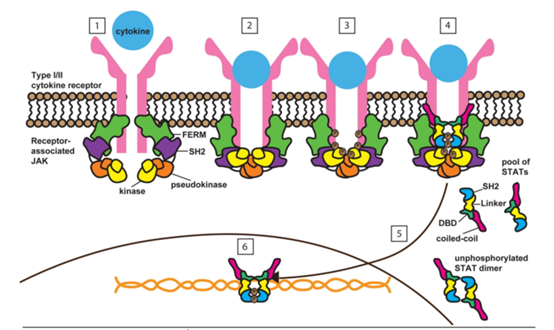
Figure 1. molecular biology of JAK [1].
How does JAK fulfill its role? When relevant signaling molecules, including various cytokine receptors (e.g., interferon receptor, interleukin receptor, etc.) are bound by the corresponding ligands (cytokines), JAKs are activated and phosphorylate the receptor, providing sites for signal transduction and binding of transcription activating proteins (STATs). Subsequently, JAKs further phosphorylate STATs, triggering a series of signaling events that ultimately lead to altered transcription of specific genes. In this process, cytokine receptors can be broadly classified into classes I and II, with about 50+ using JAKs as downstream intracellular signaling pathways.
Meanwhile, Janus kinases can exist in different combinations, for example, JAK1 and JAK3, JAK1 and JAK2, JAK1 and JAK3, and JAK1 and Tyk2 proteins can be combined, and these different combinations, combined with different receptors, allow Janus kinases to play a wide range of roles in biology, including on tissue development, cell proliferation, metabolism, infection, inflammation and tumorigenesis, especially on immune function (Figure 2) [2].
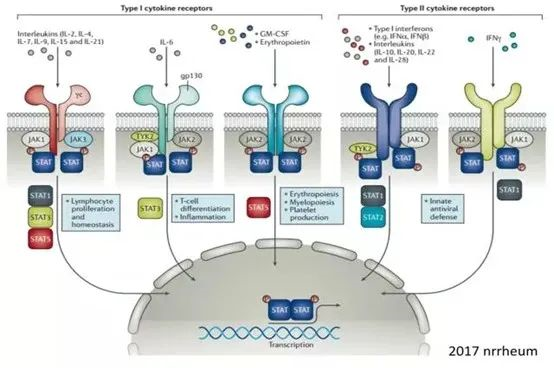
Figure 2. JAK/STAT signaling pathway as the main axis of immune/inflammatory diseases [2].
Different subtypes of JAK have their unique biological functions. In general, JAK1 is widely expressed in many cell types and plays a key role in cell growth, differentiation, inflammatory response and immune response; the function of JAK2 mainly affects bone marrow hematopoietic cells and blood cells, and is closely associated with the immune function of the body; JAK3 is mainly expressed in immune cells, and is essential for the activation, proliferation and differentiation of T and B cells, and plays a key role in the immune response and the formation of immune memory. Tyk2 is expressed in a wide range of cell types and is important for immune responses to bacteria and viruses, allergic reactions and self-immune diseases (Figure 3) [3]. If a compound is able to inhibit JAK kinase activity, its impact on immune function will undoubtedly be extensive.
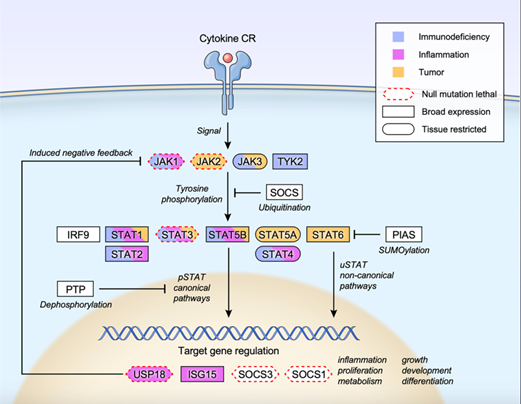
Fig. 3. Functions of different subtypes of JAK [3].
2 Progress in JAK inhibitor drug development and route of administration research
2.1 Progress of JAK inhibitor drug development
The JAK-STAT pathway was discovered in the 1990s and was recognized by Pfizer in 1993 as a potential drug target for a variety of diseases. In 2012, the U.S. Food and Drug Administration (FDA) approved tofacitib, a JAK inhibitor, for the treatment of rheumatoid arthritis, and achieved good results in clinical practice, but the multiple side effects triggered by its use as a broad-spectrum JAK inhibitor caused FDA concern. Therefore, the FDA required further studies on the safety of tofacitib's use after its introduction to the market.
After two to three years of research, the FDA issued a black box warning on tofacitib due to the finding that it may cause some serious side effects in clinical use. At the same time, the FDA believes that the JAK inhibitor class of drugs may all have potentially similar side effects, and has therefore issued black box warnings for all of these drugs. The major risks listed in the black box warnings include the risk of cardiovascular events (e.g., myocardial infarction, stroke, and possibly death), thrombosis, tumors, and serious infections (Figure 4). The issuance of the black box warning has left a negative impression of non-selective JAK inhibitors in the eyes of the public, i.e., these drugs are highly effective but have questionable safety [4].
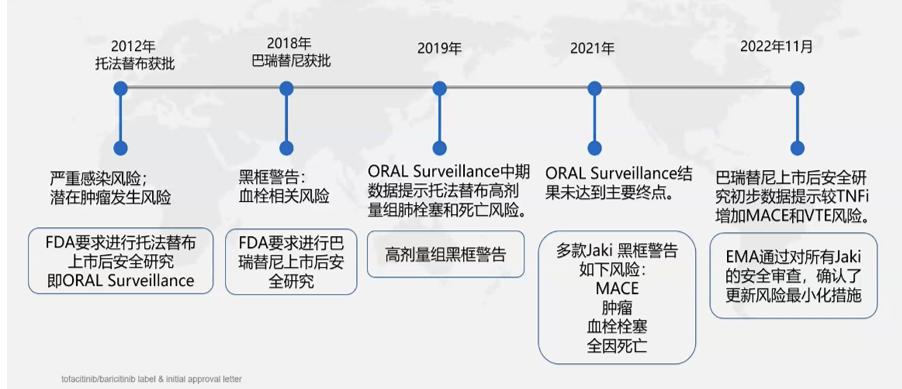
Fig. 4. Progress in JAK inhibitor drug development [4].
In the pharmaceutical industry, when the safety of an effective drug target is in question, a common solution strategy is to enhance the drug's selectivity in order to avoid the possible effects of a non-selective drug on other normal biological functions, which can cause multiple side effects. This explains why the selectivity of first-, second- and third-generation JAK inhibitors has been progressively improved as R&D progresses. Meanwhile, globally marketed JAK inhibitors now cover a wide range of disease areas such as dermatology, myelofibrosis, rheumatoid arthritis, psoriasis, pemphigus, and pemphigus vulgaris (Figure 5).
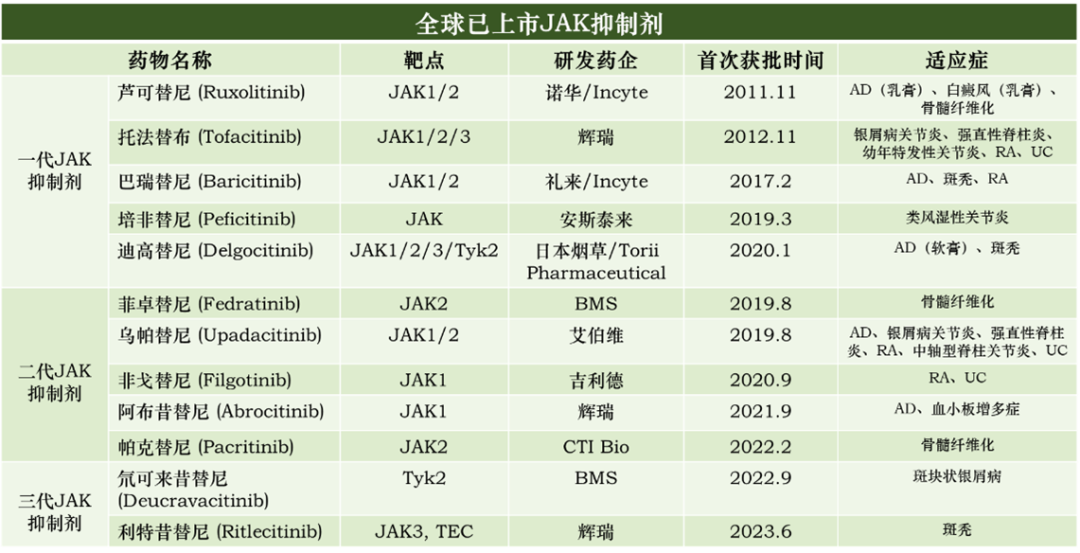
Figure 5. globally marketed JAK inhibitors (as of June 25, 2023)[5]
2.2 Characteristics and advantages of topical routes of administration of JAK inhibitors
Another solution to improve drug safety is to change the route of administration. If a suitable indication is found, the drug can have a therapeutic effect but not allow the drug to enter the bloodstream or metabolize away quickly in the body to avoid toxic side effects. During the development of new drugs, some compounds are selected that can be rapidly metabolized by entering the bloodstream. In addition, some formulation techniques are used to minimize the entry of compounds into the bloodstream, thus avoiding systemic toxic side effects and circumventing the various toxic side effects mentioned in the black box warning.
There are many types of topical medications, including eye drops (for topical use in the eyes), ointments (for topical use on the skin), nasal sprays (for topical use in the nose) and transdermal absorbed drugs. Pucci Pharma mainly produces topical medications for the skin, which is a different strategy from transdermal absorbed drugs, which are intended to work by allowing the drug to pass through the skin into the bloodstream (Figure 6). However, in order to avoid toxic side effects, the goal of the JAK inhibitors developed by the Pucci team is to minimize the entry of the drug into the bloodstream while allowing the JAK inhibitors to exert their clinical effects topically on the skin.

Figure 6. Characteristics and advantages of the topical route over other routes of administration.
3. Pukki Pharma: Innovative topical drugs focusing on immunomodulatory and inflammatory targets
After years of development and accumulation, PUKI Pharma has successfully established a pipeline of innovative drugs that specialize in skin, ophthalmic and respiratory diseases. Pucci's first compound is the JAK1/2 inhibitor PG-011 (pemetrexedinib). This is a widely validated target, and after discovering the potential of PG-011 as a preclinical candidate compounds (PCC), the R&D team conducted a series of extension studies for indications and formulations [6].
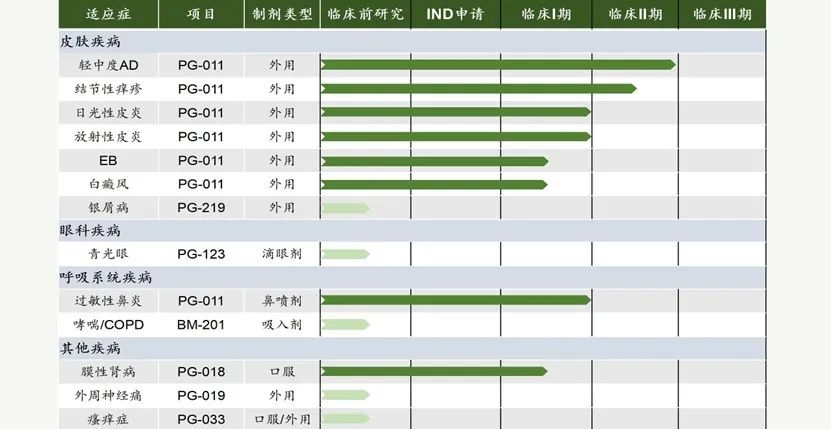
Figure 7. Pucci Pharma R&D pipeline
3.1 Topical PG-011 Gel
PGI Pharma's fastest progressing pipeline is the treatment of atopic dermatitis (AD), a chronic, recurrent inflammatory dermatosis characterized by dryness of the skin, intense itching, and rashes, which severely affects patients' daily lives. In China, more than 20% of dermatology outpatients are AD patients, with a prevalence rate of nearly 10% in adults and up to 20% in children, constituting a large patient group. For AD, topical skin medication is still the primary treatment.
As shown in Figure 8, this is the pathogenesis of AD at the cellular level, both early and late. Overall, the pathogenesis of AD is mainly an immune response mediated by Th2 cells, while other immunogenic cells such as Th17 and Th22 also play a role in the later stages of the disease [7].
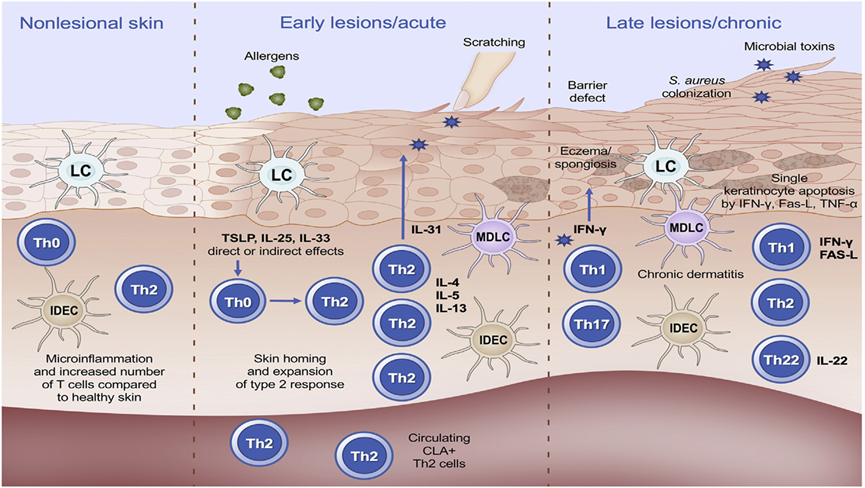
Fig. 8. Role of T cells and dendritic cells in atopic dermatitis [7].
Pukki Pharma's PG-011 drug shows significant advantages in comparison with competitors. Firstly, PG-011 has a broader inhibitory effect on inflammatory factors; secondly, as it avoids entering the bloodstream, it effectively reduces the toxic side effects it may cause; furthermore, PG-011 has the ability to promote skin collagen production and potential skin repair. Not only that, PG-011 also possesses molecular properties suitable for topical application on the skin and with the formulation characteristics of gel, thus improving patient compliance (Figure 9) [6].
3.2 PG-011 Nasal Spray
The Pucci Pharma team utilized the same compound, PG-011, to develop a nasal spray designed to treat seasonal allergic rhinitis and meet the clinical needs of a wide range of patients. The nasal spray has completed Phase I clinical trials. In the trial, the nasal spray showed an excellent nasal dose-systemic exposure (Cmax and AUC) linear relationship, which could reach steady state quickly and did not accumulate in the body, indicating a controllable dose and safety with low blood entry. Meanwhile, the nasal spray has excellent local tolerability.
4. Conclusion
Currently, PUCHI has established a product pipeline featuring topical drugs around immunomodulatory and inflammatory targets, with indications covering a wide range of dermatological diseases such as AD, nodular itchy rash, herpetic epidermolysis bullosa, psoriasis, as well as allergic rhinitis, peripheral neuralgia, glaucoma, etc. In recent years, PUCHI has been able to build up a unique product pipeline through unique and innovative approaches. In recent years, through its unique differentiated layout strategy, Pukki Pharma has been actively searching for genuine unmet clinical needs and is committed to developing innovative medicines with real clinical value, thus benefiting more patients.
Reference:
Nat Rev Drug Discov 17:78, 2017
Nat Rev Rheumatol 13, 234-243, 2017
Adapted from Cell 185:3857, 2022
Tofacitinib/baricitinib label & initial approval letter
Data source: Once Upon a Time Pharmaceuticals
Source: Pucci Pharma
Allergology International 69; 2, 204-214, 2020